General Guidance
Download / Print Section as PDFPlease note that the recommendations in this guideline application refer to Adult patients only.
General Guidance on Antimicrobial Prescribing
General Guidance on Antimicrobial Prescribing
- Please document the indication and intended duration of antimicrobial therapy in the medical record. Review all antimicrobial therapy daily.
- These guidelines are intended for empiric use only i.e. pathogen unknown. Once pathogen(s) identified antimicrobial therapy should be changed in line with antimicrobial susceptibility test results. Contact microbiology team for advice on de-escalation or rationalisation to targeted therapy . It is the responsibility of the person/team ordering laboratory tests to follow up on the test results.
- Piperacillin-tazobactam and co-amoxiclav provide good anaerobic cover. Concurrent metronidazole is NOT required unless there is gross faecal contamination e.g. faecal peritonitis, or there is an undrained collection or abscess. Treatment of aspiration pneumonia does not require addition of metronidazole to either of these antibiotics.
- Antimicrobials such as ciprofloxacin, levofloxacin, metronidazole, linezolid, clindamycin, and fluconazole have excellent oral bioavailability and the oral route should be selected where possible when using these agents. IV formulations should only be used where adequate absorption from the GIT is a concern or oral medications are not tolerated or advised.
- Oral switch – consider changing to oral antimicrobials when patient has been afebrile with infection parameters settling for 48 hours, and where absorption from the GIT is considered sufficient. Oral antimicrobials are generally NOT appropriate for use in the treatment of acute bacterial meningitis, infective endocarditis, febrile neutropenia or acute osteomyelitis and septic arthritis.
- For oral switch guidelines please see IV to PO Switch section. Oral switch is usually to the oral formulation of the same antimicrobial where available. Exceptions include IV benzylpenicillin which should be changed to PO amoxicillin as absorption of oral penicillin is variable.
- Penicillin allergy Obtain & document exact nature of allergy.
- In IgE-mediated (e.g. anaphylaxis, angioedema, immediate urticarial rash) allergy or a history of severe reaction to penicillin (eg. erythema multiforme/Steven-Johnson Syndrome/TEN), avoidance of all beta-lactam antibiotics is advised.
- In non-IgE-mediated allergy or non-severe reaction to penicillin such as a mild-moderate rash (NOT erythema multiforme/Steven-Johnson Syndrome/TEN), a cephalosporin should be considered. Macrolides such as erythromycin are often NOT a good substitute for empiric use in serious infections due to limited spectrum of activity and rising resistance rates.
- Flucloxacillin and other beta-lactams such as co-amoxiclav, piperacillin-tazobactam, cephalosporins and meropenem do NOT cover MRSA. Vancomycin or teicoplanin can be used to treat MRSA infections if required.
- Clostridioides difficile infection can be associated with the use of all antibiotics. There is a particular risk recognised with the use of fluoroquinolones (e.g. levofloxacin and ciprofloxacin), clindamycin and 3rd generation cephalosporins such as ceftriaxone and other broad spectrum antibiotics. Concurrent administration of these agents and prolonged courses of all broad-spectrum antibiotics should be avoided where possible.
- Note on fluoroquinolones : The use of fluoroquinolones (eg. ciprofloxacin, levofloxacin) is associated with a risk of disabling, long-lasting and potentially irreversible side effects (including tendon damage, QT prolongation, neuropathies, and neuropsychiatric effects). Older patients, those with renal impairment, solid organ transplantation or on systemic corticosteroids are at an increased risk of tendon damage with these agents. Please read the HPRA Drug Safety Alert issued in 2018 and the HPRA Drug Safety Newsletter issued in 2023 highlighting restrictions on use of fluoroquinolones (eg. ciprofloxacin, levofloxacin). Quinolones also associated with an increased risk of C. difficile infection.
- Note that sodium fusidate and rifampicin should never be used as monotherapy for the treatment of infections caused by Staphylococcus aureus as resistance to these agents can rapidly develop on treatment.
- Note on Macrolides (eg erythromycin, clarithromycin): This class of antibiotics has a number of side effects, interactions and contraindications that should be taken into account when prescribing. Particular caution should be taken with use in older patients and in those with a history of cardiac disease.
- Please prescribe exact doses where weight-based calculations are required such as when using Gentamicin or Vancomycin. Failure to do so can lead to medication being administered at incorrect dosages with the potential for unintended adverse outcomes.
NB: The prescriber should always check prescribing information such as cautions, contraindications, interactions and side effects when considering antimicrobial therapy. Ensure information on antimicrobial prescribing, including risks and side effects associated with antimicrobial treatment, is available to patients or their legal guardians.
Disclaimer:
Whilst every effort has been made to ensure the accuracy of the information and material contained in this document, errors or omissions may occur in the content. We acknowledge that new evidence may emerge that may overtake some of these recommendations. The document will be reviewed and revised annually. Prescribers should ensure that the correct drug and dose is prescribed, as is appropriate for each individual patient. References that should be used in conjunction with these guidelines include the British National Formulary (BNF) and the drug data sheets and SPCs. Clinical guidelines are guidelines only and the interpretation and application of the guidelines remains the responsibility of the individual clinician.
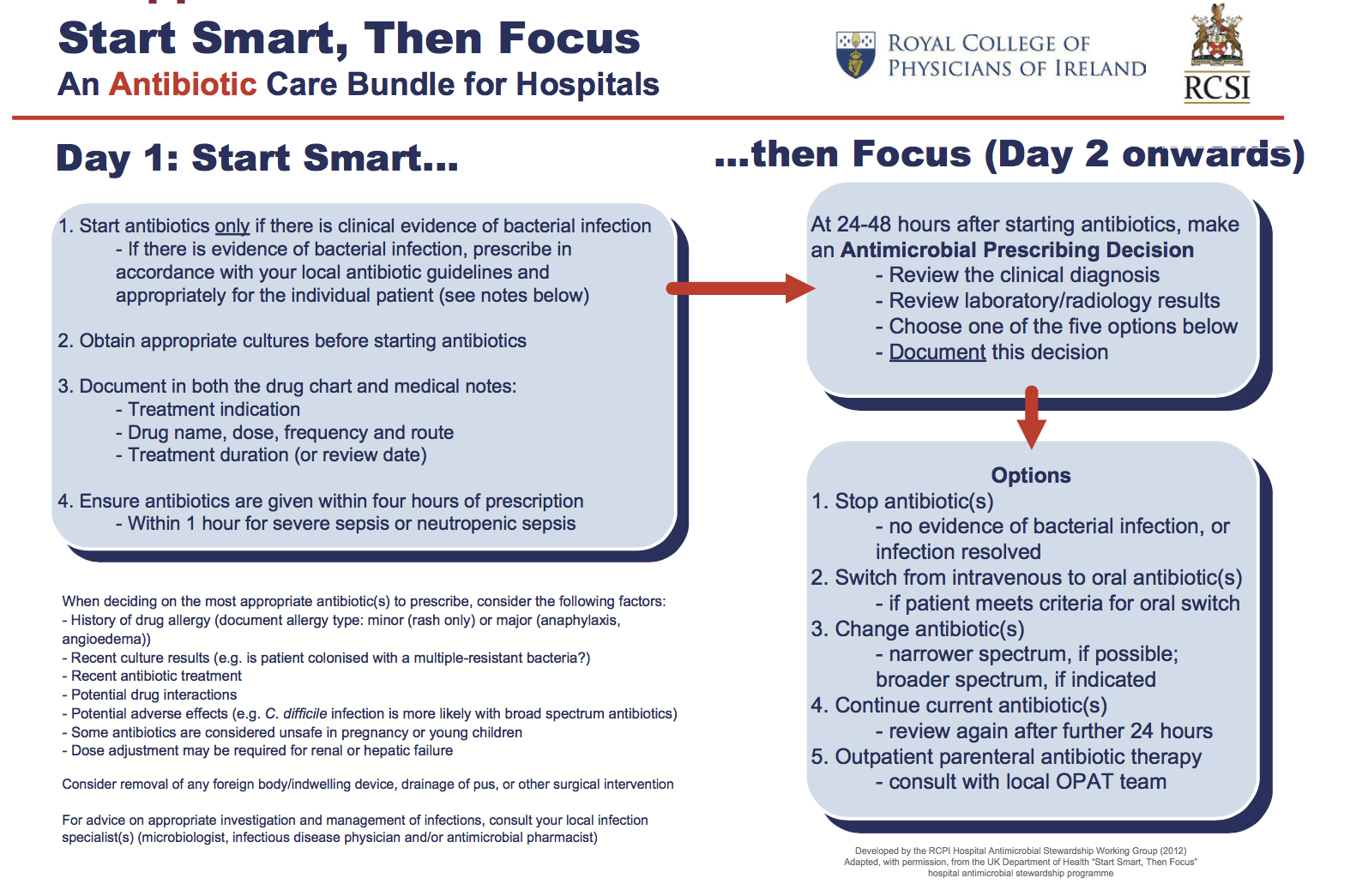
Start Smart, Then Focus Care Bundle
Start Smart, Then Focus Care Bundle
Guidelines for Consulting the UHW Clinical Microbiology Advisory Team (CMAT)
The On-Duty Clinical Microbiology Advisory Team (CMAT) can be contacted on the contact numbers provided (9.30 - 17.30 Monday to Friday). A consultant out-of-hours-service is available for urgent clinical advice outside of these hours.
Prior to contacting the team please have the following 3 actions completed:
- Review the Guidelines for Empiric Use of Antimicrobials in Adults – many queries including empiric therapy by condition, treatment algorithms and therapeutic drug monitoring can be answered here.
- Ensure the patients’ previous microbiology results have been reviewed and summarised and available for the consultation.
- Provide the CMAT team member with an ISBAR summary – please see below
C-MAT Consultation Modified ISBAR
Identify
1. Yourself, 2. Patient, Name, MRN, DOB, Ward
Situation
Summarise main issues related to sepsis / infection Include other relevant key issues in presenting complaint
Background
LOS in hospital lead up to this point – course in hospital, procedures done and dates, relevant test results, current antimicrobial therapy
Assessment
Outline relevant details of your clinical assessment– general impression, signs and symptoms, temperature, BP, NEWS score etc.; Laboratory findings – e.g. WCC, CRP, Lactate, RFTs, LFTs; Other such as relevant imaging results (Echo, CT, MRI, Bone Scan, Other)
Results of other relevant investigations available / pending
Recommendations
Based on this consultation, accurately document the further recommendations from the C-MAT team which may include for example:
-
Further tests
- Micro (blood cultures, swabs, fluids, tissue, stool, sputum, urine, serology, CSF, other)
- Lab (WCC ,CRP, Lactate, U/E, LFTs, antibiotic drug levels, Other)
- New / Further Imaging (Echo, CT, MRI, Bone Scan, Other)
-
Antimicrobial therapy
- Recommendations on antimicrobial therapy, choice of agent(s), dose optimisation, proposed length of treatment.
Restricted/Reserve Antimicrobials
Criteria for restricting antimicrobials include potential toxicity, antimicrobial resistance, inappropriate use and high cost. The WHO AWaRe Classification of antimicrobials divides into three groups, AWaRe- Access , Watch and Reserve.
Indication for therapy and any discussions/advice from the Clinical Microbiologist should be documented accurately in patient’s medical record.
Please refer to South East Acute Hospitals Guidelines for use of Reserve and Restricted Antimicrobials (ASG002) for further details.
A ccess; Unrestricted agents
Although not subject to specific restrictions, antimicrobial agents should be prescribed judiciously and only if clinically indicated.
W atch: Restricted agents
Agents that should be conserved for situations in which use is clearly justifiable and not freely available to all. It is recommended that they are only prescribed when it is in line with the recommendations of the local antimicrobial guidelines or following discussion with the Clinical Microbiologist.
R eserve: Reserve agents
Last-line agents that should be reserved for use only when other agents cannot be expected to be effective. They should only be prescribed when recommended by a Consultant and following discussion with the Clinical Microbiologist.
|
Watch: Restricted Antimicrobials |
Reserve: Reserve Antimicrobials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Antifungals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Antivirals |
|
|
|
References:
- HSE AMRIC Antimicrobial Stewardship guidance for all healthcare settings, August 2022 Antimicrobial Stewardship Guidance for all Healthcare Settings
MRSA
MRSA
MRSA (Methicillin Resistant Staphylococcus aureus )
MRSA = S. aureus that is resistant to Flucloxacillin and most other β-lactam antibiotics (e.g. co-amoxiclav, piperacillin-tazobactam, ceftriaxone).
When to suspect MRSA infection:
1. Compatible clinical presentation
- An infection usually caused by S. aureus such as skin and soft tissue infections, line (CVC or PVC) related infections, bone and joint infections, sepsis, and acute infective endocarditis
AND
2. At-risk patient
-
A history of MRSA colonisation or infection.
- Please check medical notes, laboratory results and any current Infection Control alerts such as Laboratory SIF, IPMS Infection Control alert, etc.), or
-
Risk factors for MRSA colonisation or infection such as:
- Recent hospitalization (within 12 months), or
- Transfer from another hospital or residential care facility.
Please refer to most recent Policy on Control and Prevention of Methicillin Resistant Staphylococcus aureus (MRSA) in Acute Hospitals in the HSE/SE for additional information.
If MRSA infection is suspected, consider the addition of a glycopeptide (vancomycin or teicoplanin) to the empiric antimicrobial regime.
- Consider stopping glycopeptide if based on culture results there is NO evidence of MRSA colonisation or infection.
MRSA eradication therapy:
- Please refer to most recent edition of: Policy on Control and Prevention of Methicillin Resistant Staphylococcus aureus (MRSA) in Acute Hospitals in the HSE/ SE.
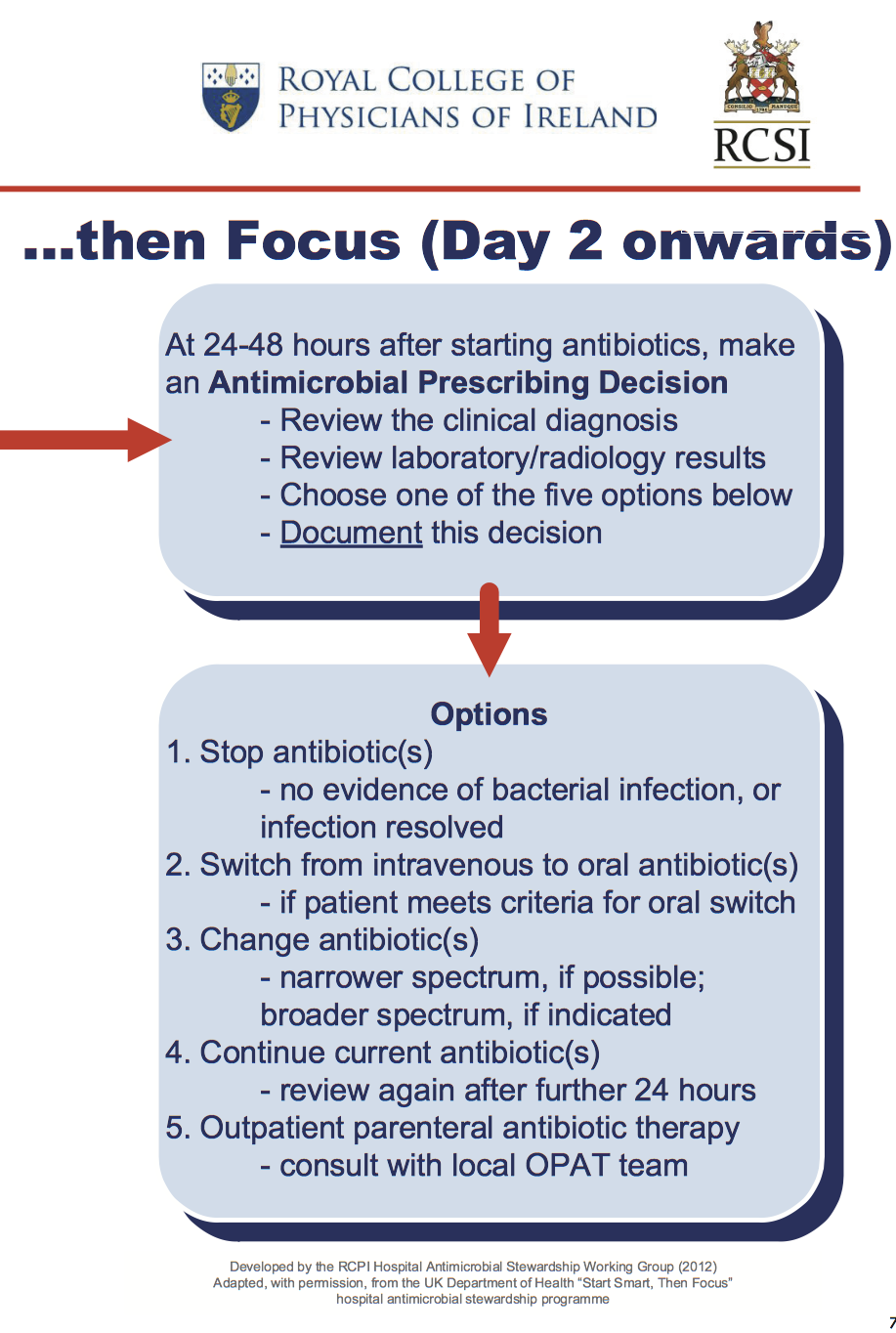
RCPI/RCSI Start Smart Then Focus
Start Smart, Then Focus: An Antibiotic Care Bundle for Hospitals

Antimicrobial Susceptibility
Antimicrobial Susceptibility Definition
European Committee on Antimicrobial Susceptibility Testing (EUCAST) has made significant changes to the interpretation of antimicrobial susceptibility testing, in particular, ‘I’ now refers to ‘Susceptible, Increased Exposure’ and no longer indicates ‘intermediate’ susceptibility or uncertain therapeutic effect.
|
Susceptible |
S |
Susceptible, standard dosing regimen |
|
I |
Susceptible, increased exposure* (increased dose) |
|
|
Resistant |
R |
Resistant |
The following revised definitions of ‘S’, ‘I’ and ‘R’ now apply:
‘S’ Susceptible, standard dosing regimen : A microorganism is categorised as ‘S’ when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent.
‘I’ Susceptible, increased exposure *: A microorganism is categorised as ‘I’ when there is a high likelihood of therapeutic success because exposure to the (antimicrobial) agent is increased by adjusting the dosing regimen or by its concentration at the site of the infection.
‘R’ Resistant : A microorganism is categorised as ‘R’ when there is a high likelihood of therapeutic failure even when there is increased exposure.
*Exposure is a function of how the mode of administration, dose, dosing interval, infusion time, as well as distribution and excretion of the antimicrobial agent will influence the infecting organism at the site of infection.
Antibiotic dosing according to SITE OF INFECTION:
***For complicated/deep-seated infections such as meningitis, infective endocarditis, implant infections or systemic/severe infections, high dose systemic antimicrobials are required to achieve therapeutic success. Please discuss with Clinical Microbiologist for further advice.***
Frequently asked Questions: What does ‘I’ Susceptible, increased exposure mean?
Common examples of increased dose regimens for specific bacteria/antibiotics
Pseudomonas aeruginosa
- Will only be reported either as ‘I’ Susceptible, increased exposure or ‘R’ resistant to commonly use antibiotics i.e. Piperacillin/Tazobactam, Ceftazidime, Aztreonam and Ciprofloxacin. There is no longer ‘S’ category for these antibiotics for P. aeruginosa.
- ‘ I’ Susceptible, increased exposure : infection can be treated with high dose antimicrobial regimens i.e. an increased dosing interval of Piperacillin/Tazobactam of 4.5g QDS IV or increased dose of Ceftazidime 2g TDS IV (dose adjustment are required for renal/liver impairment). It does NOT indicate escalation to Meropenem (restricted agent).
Haemophilus influenzae (ORAL amoxicillin and ORAL Co-amoxiclav)
- Will only be reported either as ‘I’ Susceptible, increased exposure or ‘R’ resistant to ORAL Amoxicillin or ORAL Co-amoxiclav. There is no longer ‘S’ category for ORAL Amoxicillin or ORAL Co-amoxiclav for H. influenzae .
- ‘I’ Susceptible, increased exposure to ORAL Amoxicillin: infection can be treated with increased dose of oral Amoxicillin 750mg-1g TDS PO or standard dose of IV Amoxicillin. It does NOT indicate escalation to Co-amoxiclav.
- ‘I’ Susceptible, increased exposure to ORAL Co-amoxiclav: infection can be treated with increased dose of Co-amoxiclav 875/125mg TDS PO, or Co-amoxiclav 625mg TDS PO PLUS Amoxicillin 500mg TDS PO or a standard dose of IV Co-amoxiclav. It does NOT indicate escalation to IV Piperacillin/Tazobactam.
Antibiotic dosing according to SITE OF INFECTION:
***For complicated/deep-seated infections such as meningitis, infective endocarditis, implant infections or systemic/severe infections, high dose systemic antimicrobials are required to achieve therapeutic success. Please discuss with Clinical Microbiologist for further advice.***
Common examples of increased dose regimens when susceptibility reported as ‘I’ Susceptible, increased exposure.
|
Bacteria |
Antibiotics |
Increased dose regimen |
Comments |
|
Pseudomonas aeruginosa |
Piperacillin/ Tazobactam IV |
4.5g QDS |
Consider 3 hour infusion for critical illness |
|
Ceftazidime IV |
2g TDS |
|
|
|
Ciprofloxacin PO |
750 mg BD |
HPRA Drug Safety Alert 2018 & 2023 Caution with use |
|
|
Ciprofloxacin IV |
400mg TDS |
||
|
Haemophilus influenzae |
Amoxicillin PO |
750mg-1g TDS |
|
|
Co-amoxiclav PO |
Option 1: Co-amoxiclav 625mg TDS PLUS Amoxicillin 500mg TDS
Option 2: Co-amoxiclav 875/125mg TDS |
Options 1 or 2 depend on antibiotic stock/availability. Option 1 would lead to an increase in Amoxicillin consumption |
|
|
Other |
Please consult Clinical Microbiologist |
||
Note: Dosing recommendations are for adult patients with normal renal/liver function. Higher than standard dosing of some antibiotics may be required for severe infections. Please contact the Clinical Microbiology Team for further advice if required. Table adapted from: Scottish Antimicrobial Prescribing Group (SAPG) and the Scottish Microbiology and Virology Network (SMVN).
High Tech Antimicrobials
On discharge from hospital, the following antimicrobials must be prescribed via the High Tech Scheme on a High Tech prescription or via the High Tech Hub online :
• Fidaxomicin ( tablets & suspension )
• Linezolid ( tablets & suspension )
• Voriconazole ( tablets & suspension )
• Posaconazole ( tablets & suspension )
• Isavuconazole ( capsules )
• Valganciclovir ( tablets & suspension )
• Tobramycin ( nebules )
• Aztreonam ( nebules )
• Colistin ( inhaler )
Note: If High Tech antimicrobials are prescribed on a standard discharge prescription , the community pharmacy will not be able to order or dispense the medication, resulting in unnecessary delays and/or interruptions to treatment.